Abstract
Introduction: Acute myeloid leukemia (AML) is the most common acute leukemia of adults, with median age at diagnosis of 68 years. Mutations in FMS-like tyrosine kinase-3 internal tandem duplication (FLT3-ITD) are associated with a higher risk of relapse and inferior overall survival (OS) in younger patients treated with intensive chemotherapy, but the prognostic relevance among older patients treated with non-intensive approaches is not well established. As well, the role of nucleophosmin (NPM1), and its interaction with FLT3-ITD status and allelic ratio (AR) remains unclear among elderly. We aimed to assess the prognostic impact of FLT3-ITD and NPM1 mutations in older/unfit newly diagnosed AML patients treated with non-intensive front-line therapies.
Methods: We conducted a retrospective analysis that included 707 patients treated with different non-intensive regimens in the PETHEMA AML registry, between 2007 and 2020. FLT3-ITD and NPM1 mutations were studied by fragment length analysis. The karyotype information was available in 615 patients. Associations of demographic and clinical parameters in patients harboring FLT3-ITD and NPM1 mutations was performed using Mann-Whitney-U-test for continuous and Chi-square test for discrete variables. Overall survival (OS) and relapse curves were constructed using Kaplan-Meier and a log-rank test. All analyses were performed by SPSS.
Results: The median age was 74 years (range: 43-91), and 410 patients (58%) were male. ECOG status was 0/1 in 474 patients (72%) and 2 in 124 patients (18.9%). FLT3-ITD mutation was identified in 99 patients (14%). When available, 33 patients (40.7%) showed FLT3-ITDlow (AR <0.5). NPM1 mutation was detected in 144 patients (20%). Karyotype was abnormal in 301 patients (42.7%). Regarding the frontline therapy 328 (46.3%) patients received hypomethylating agents (HMA); 353 (49.9%) fludarabine + low dose cytarabine (LDAC) (FLUGA); 11 (1.6%) fludarabine + LDAC + oral idarubicin (FLAG-IDA Lite); and 15 (2.1%) LDAC. Mortality at 2 years was 77.6% and median OS 6 months.
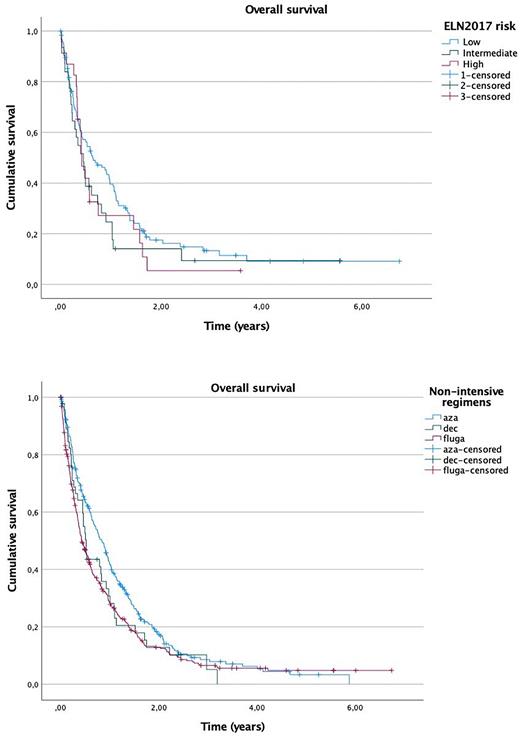
FLT3-ITD patients (N=99) showed a non-significant difference in OS compared to FLT3 wild-type (FLT3WT) patients (N=608): median OS 5 vs 7.3 months (p=0.17). There were no differences when comparing low and high AR: FLT3-ITDlow (N=34) vs FLT-ITDhigh (N=47): median OS 5.5 vs 4.9 months (p=0.64). Survival in NPM1-mutated patients (N=144) was similar than in NPM1WT patients (N=519): median OS 7.2 vs 6.8 months (p=0.24). OS according to ELN 2017 risk stratification, considering only NPM1 and FLT3-ITD mutations, was similar in subgroups low risk (N=117), intermediate risk (N=31), high risk (N=23): median OS 7.6 vs 5.4 vs 7.2 (p=0.26) (Fig. 1).
In the subgroup of normal karyotype (N=314), FLT3-ITD patients (N=64) showed a trend towards lower survival as compared to FLT3WT patients (N=249): median OS 5.4 vs 10.9 months (p=0.058), with no impact of AR: FLT3-ITDlow (N=19) vs. FLT3-ITDhigh (N=32) median OS 5.8 vs 4.8 months (p=0.73). NPM1 mutated patients (N=102) showed a non-significant difference with NPM1WT patients (N=196): median OS 7.2 vs 10.3 months (p=0.66). OS according to ELN 2017 considering only NPM1 and FLT3-ITD status was similar in subgroups: low risk (N=80), intermediate risk (N=24), high risk (N=12): median OS 2.3 vs 11 vs 2.5 months, respectively (p=0.64). AML patients treated with azacitidine (N=281) had longer median OS of 10 months (p=0.005; 95% CI 1.03 to 1.37) vs decitabine (N=47) median OS 6.1 months and FLUGA median OS 5 months (N=352) (Fig. 2).
Conclusions: Data from this large cohort of AML patients treated with non-intensive regimens underlines the limitations of the ELN 2017 risk stratification in this setting. Nonetheless, there was a trend towards worse OS in patients with normal karyotype and FLT3-ITD mutation (irrespectively of the allelic burden) as compared to FLT3WT patients.
Figure 1. Overall survival according to ELN 2017 risk stratification
Figure 2. Overall survival regarding non-intensive regimens.
Disclosures
Alonso:Incyte: Research Funding; Pfizer: Research Funding; Astellas: Research Funding; Celgene: Research Funding. Ramos:BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria; Novartis: Honoraria; Abbvie: Honoraria; Pfizer: Honoraria; Astellas: Consultancy, Honoraria; Sandoz: Honoraria; Jazz: Honoraria. Bergua Burgués:Incyte: Research Funding; Pfizer: Research Funding; Astellas: Research Funding; Celgene: Research Funding. Montesinos:Kura Oncology: Consultancy; Beigene: Consultancy; Nerviano: Consultancy; Jazz Pharmaceuticals: Consultancy, Research Funding, Speakers Bureau; Pfizer: Consultancy, Research Funding, Speakers Bureau; Takeda: Consultancy, Research Funding; Incyte: Consultancy; Astellas: Consultancy, Speakers Bureau; Novartis: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding, Speakers Bureau; Abbvie: Consultancy, Research Funding, Speakers Bureau; Gilead: Consultancy, Speakers Bureau; Menarini/Stemline: Consultancy, Research Funding; Otsuka: Consultancy; Ryvu: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal